Trends in ambulatory cardiology consultations for suspected myocarditis after COVID-19 vaccination
- Ottieni link
- X
- Altre app
Trends in ambulatory cardiology consultations for suspected myocarditis after COVID-19 vaccination
- Holger Eggebrecht,
- Philipp Breitbart,
- Alexander Koch,
- Bernd Nowak,
- Claudia Walther,
- Thomas Voigtländer,
- Christoph Liebetrau,
- Rajendra H. Metha &
- Axel Schmermund
- In Germany, vaccination against coronavirus disease 2019 (COVID-19) was approved on December 21st 2020 and the first patient treated on December 26th. On May 7th 2021, the European Medicines Agency (EMA) announced an official investigation of case reports of myopericarditis in temporal relation to COVID-19 vaccination [1,2,3], which was updated on June 11th [4]. Since then, we noted increasing requests from General Practitioners for urgent cardiologic consultation of patients with suspected myocarditis after COVID-19 vaccination. All patients were scheduled within the 2 following business days and underwent standardized cardiologic assessment including electrocardiography (ECG), echocardiography, and determination of NT-proBNP and high-sensitive troponin T. Cardiac magnetic resonance (CMR) was performed at the discretion of the treating cardiologist. Statistical analysis was performed using IBM SPSS Statistics 27.0. Continuous variables are presented as mean and 95% confidence interval [CI], and categorical variables are represented as frequencies and percentages. Comparisons were performed with Student’s t test and with the Chi-square test for categorical variables. A two-sided p value of ≤ 0.05 was considered statistically significant.
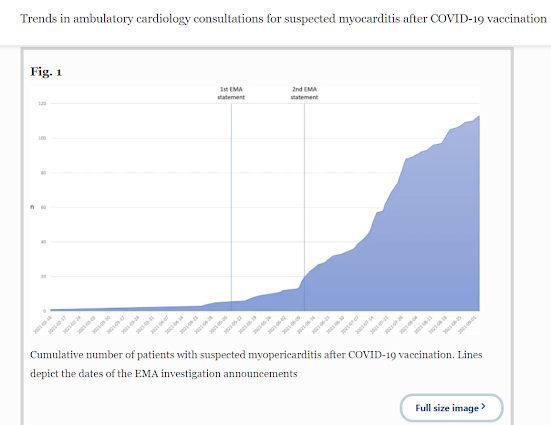
- Between December 27, 2020 and September 3, 2021, a total of 113 patients (age: 45.9 years, 95% CI 43.2–48.7; 44% females) were referred to our cardiology practice for suspected myopericarditis after COVID-19 vaccination. Cumulative number of patients increased exponentially after the EMA investigation (Fig. 1). Forty-nine (43%) patients presented after the first vaccine dose (delay: 25.5 days, 95% CI 18.8–32.1), while the remaining 64 (57%) patients were referred after the second dose (28.6 days; 95% CI 20.7–36.5). Vaccines included BNT162b2 mRNA (Pfizer–BioNTech) (n = 85, 75%), mRNA-1273 (Moderna) (n = 13, 12%), ChAdOx1 nCoV-19 (AstraZeneca) (n = 5, 4%), and Ad26.COV2.S (Johnson&Johnson) (n = 6, 5%). Four patients (4%) received a combination (ChAdOx1 nCoV-19, followed by BNT162b2 mRNA). Symptoms reported after vaccination that triggered evaluation included chest pain (n = 68, 60%), shortness of breath (n = 36, 32%), palpitations (n = 41, 36%), fatigue (n = 25, 22%), and/or reduced exercise tolerability (n = 30, 27%).
- ..
- Given the increasing number of vaccine recipient reporting cardiac symptoms and rarity of confirmed myopericarditis makes routine use of CMR for diagnosis of myocarditis post-COVID-19 vaccination cost-prohibitive. However, particularly in symptomatic young boys and adolescents who carry a significantly increased risk of myopericarditis after mRNA vaccination [2], CMR remains important to rule out vaccine-associated myocardial injury.
- FULL TEXT HERE:
- Ottieni link
- X
- Altre app



Commenti
Posta un commento